Your Location:Home > Products > 3-Hydroxy-4-amino-butyric acid
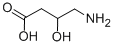

CAS No:352-21-6
MF:C4H9NO3
Appearance:white or off-white crystalline powder
Specification:98.5%
Package:1/5/10/25kgs/drum
Supply Capacity:Tons
Synonyms:3-Hydroxy-4-amino-butyric acid; DL-4-Amino-3-hydroxybutyric acid; DL-gamma-Amino-beta-hydroxybutyric acid; 4-Amino-3-hydroxybutanoate;4-amino-3-hydroxy-butyricaci;gamibetal;gamma-amino-beta-hydroxybutyricacid;Aminohydroxybutyricacid;DL(-3-hydroxy-4-amino butyric acid;alpha-Hydroxy-gamma-aminobutan;DL-4-AMINO-3-HYDROXY BUTYRIC ACID [GABOB]; Gamma-Aminobutyric Acid,"gamma-aminobutyric acid; GABA; 56-12-2; Piperidic acid; Piperidinic acid; Aminalon; butanoic acid, 4-amino-; Gaballon; Gammalon; Gammalone; Gammasol; Mielomade; Gamarex; Gammar; Butyric acid, 4-amino-; gamma-amino-n-butyric acid; gamma-Aminobutanoic acid; Immu-G; Gammagee; Gamulin; 4-aminobutyrate; omega-Aminobutyric acid; Aminalone; 4-aminobutanoate; gamma-Aminobuttersaeure; gamma Aminobutyric acid; Factor I; 4Abu; 2ACZ6IPC6I; Aminobutyric acid, gamma-; NSC-27418; CHEBI:16865; Lithium GABA; GABA, Lithium; 4 Aminobutyric Acid; 4 Aminobutanoic Acid; Therafeldamine; Theraproxen; GABA Phenolic; Therapentin-60; Theraprofen-60; Theraprofen-90; Theraproxen-90; Theratramadol-60; Theratramadol-90; Theraprofen-800; Theraproxen-500; Acid, Aminobutyric; Theracodeine-300; Therabenzaprine-60; Therabenzaprine-90; Theracodophen-650; Theracodophen-750; Hydrochloride gamma-Aminobutyric Acid; Acid, Hydrochloride gamma-Aminobutyric; gamma Aminobutyric Acid, Hydrochloride; gamma Aminobutyric Acid, Monosodium Salt; Therabenzaprine-90-5; gamma Aminobutyric Acid, Monolithium Salt; gamma-amino-butyric acid; gamma.-aminobutyric acid; CARISOPRODOL, GABA; Gaba (Gamma-Aminobutyric Acid); GABA 1519; TRAMADOL HYDROCHLORIDE, GABA; NAPROXEN, GAMMA-AMINOBUTYRIC ACID; IBUPROFEN, GAMMA-AMINOBUTYRIC ACID; PIROXICAM, GAMMA-AMINOBUTYRIC ACID; GABAPENTIN, GAMMA-AMINOBUTYRIC ACID; TRAMADOL HYDROCHLORIDE, GAMMA-AMINOBUTYRIC ACID; HYDROCODONE, ACETAMINOPHEN, gamma-AMINOBUTYRIC ACID; CYCLOBENZAPRINE HYDROCHLORIDE, GAMMA-AMINOBUTYRIC ACID; ACETAMINOPHEN, CODEINE PHOSPHATE, GAMMA-AMINOBUTYRIC ACID; HYDROCODONE BITARTRATE, ACETAMINOPHEN, GAMMA-AMINOBUTYRIC ACID; 200-258-6; 4-azaniumylbutanoate; Aminobutyric Acid; 4-aminobutyric acid; 4-Aminobutanoic acid; Mielogen; Reanal; .gamma.-Aminobutyric acid; gamma-aminobutyrate; gamma-Amino butyric acid; 3-Carboxypropylamine; 4-amino-butanoic acid; GAMMA-AMINO-BUTANOIC ACID; g-Aminobutyric acid; 4-amino butyric acid; 4-Amino-butyric acid; DF 468; CCRIS 3721; Gamma-aminobutyric acid [JAN]; 4-amino-n-butyric acid; GAMMA(AMINO)-BUTYRIC ACID; Aminobutanoic acid; .gamma.-Amino-butyric acid; 20-79-1; NSC 27418; .gamma.-Amino-N-butyric acid; EPA Pesticide Chemical Code 030802; AI3-26812; gamma-Aminobutryic acid; 4-NH2-but; 4-NH3-but; CHEMBL96; MFCD00008226; .gamma.-Aminobutanoic acid; [3H]GABA; MLS000028505; gamma-Aminobutyric acid (JAN); DTXSID6035106; Butanoic acid, 4-amino- (9CI); NSC27418; NSC32044; NSC45460; NSC51295; 4-Aminobutylate; Butyric acid, 4-amino- (7CI,8CI); NCGC00015043-07; SMR000058285; 4-AMINO-BUTYRATE; WLN: Z3VQ; 4-Aminobutyricacid; BUTANOIC ACID,4-AMINO; Chemical Name: .gamma.-Aminobutanoic acid; gamma-Aminobuttersaeure [German]; 4-aminobutanoic acid (gamma-aminobutyric acid); SR-01000075618; Acide amino-4- butyrique [French]; EINECS 200-258-6; UNII-2ACZ6IPC6I; Vigabatrin EP Impurity D; Acide amino-4- butyrique; aminobutyrate; Immuglobin; Piperidate; Piperidinate; w-Aminobutyrate; Gamma aminobutyrate; omega-Aminobutyrate; Gammalon (TN); a-Aminobutyric acid; w-Aminobutyric acid; Vigabatrin impurity D; Vigabatrin Impurity 1; gamma-aminobutyric-acid; Spectrum_000049; Tocris-0344; Aminobutyric acid,-4-; 4-Amino-n-butanoic acid; Opera_ID_1152; Spectrum2_001208; Spectrum3_001385; Spectrum4_000809; Spectrum5_001425; Lopac-A-2129; .omega.-Aminobutyric acid; cid_119; Biomol-NT_000230; bmse000340; bmse000820; bmse000871; SCHEMBL4878; Lopac0_000005; Oprea1_584567; BSPBio_002970; KBioGR_001297; KBioSS_000429; DivK1c_000616; NH2-(CH2)3-COOH; SCHEMBL442853; SCHEMBL444549; SPECTRUM1500678; SPBio_000996; GTPL1067; GTPL5410; orb1310544; SCHEMBL1498122; SCHEMBL3256936; SCHEMBL5165011; SCHEMBL6609421; SGCUT00121; DTXCID4015106; BDBM24183; HMS501O18; KBio1_000616; KBio2_000429; KBio2_002997; KBio2_005565; KBio3_002190; MSK7512; gamma-Aminobutyric acid, 99%; NINDS_000616; HMS1921C06; HMS2232P09; HMS3260A11; Pharmakon1600-01500678; 4-AMINOBUTYRIC ACID [FHFI]; BCP34675; HY-N0067; STR01523; to_000021; 4-amino-n-[2,3-3H]butyric acid; Tox21_110071; Tox21_500005; BBL008590; CCG-38515; HB0882; LMFA01100039; NSC-32044; NSC-45460; NSC-51295; NSC757426; PDSP1_001275; PDSP2_001259; s4700; SBB028179; STK301748; AKOS000119660; CS-W020704; DB02530; FA17705; KS-5273; LP00005; NSC-757426; SDCCGSBI-0049994.P004; .GAMMA.-AMINOBUTYRIC ACID [MI]; CAS-56-12-2; GAMMA-AMINOBUTYRIC ACID [MART.]; IDI1_000616; GAMMA-AMINOBUTYRIC ACID [USP-RS]; GAMMA-AMINOBUTYRIC ACID [WHO-DD]; NCGC00015043-01; NCGC00015043-02; NCGC00015043-03; NCGC00015043-04; NCGC00015043-05; NCGC00015043-06; NCGC00015043-08; NCGC00015043-09; NCGC00015043-10; NCGC00015043-14; NCGC00024546-01; NCGC00024546-02; NCGC00024546-03; NCGC00024546-04; NCGC00024546-05; NCGC00024546-06; NCGC00260690-01; BP-21452; gamma-Aminobutyric acid, BioXtra, 99%; SBI-0049994.P003; gamma-Aminobutyric acid, analytical standard; VIGABATRIN IMPURITY D [EP IMPURITY]; A0282; EU-0100005; NS00002712; ST45061580; EN300-19823; gamma-Aminobutyric acid (4-Aminobutyric acid); A 2129; C00334; D00058; D70585; Vigabatrin impurity, .gamma.-aminobutyric acid-; AB00052155_12; L000262; Q210021; SR-01000075618-1; SR-01000075618-3; SR-01000075618-6; SR-01000075618-7; BRD-K77245796-001-19-2; BRD-K77245796-003-01-6; BUTANOIC ACID,4-AMINO 4-AMINO,BUTYRIC ACID; F2191-0196; Z104475620; C5C3DC27-C105-4D18-8A52-F51978B32D24; SODIUM ALENDRONATE TRIHYDRATE IMPURITY A [EP IMPURITY]; 4-Aminobutanoic Acid;3-Carboxypropylamine;4-Aminobutyric acid;GABA; gamma-Aminobutyric acid pound(c)(3/4)?Aminobutyric acid (GABA); gamma-Aminobutyric acid, certified reference material, TraceCERT(R); InChI=1/C4H9NO2/c5-3-1-2-4(6)7/h1-3,5H2,(H,6,7; Vigabatrin impurity D, European Pharmacopoeia (EP) Reference Standard; VIGABATRIN IMPURITY, .GAMMA.-AMINOBUTYRIC ACID- [USP IMPURITY]; gamma-Aminobutyric acid, United States Pharmacopeia (USP) Reference Standard",103.120,C4H9NO2,;