Your Location:Home > Products > Dimethylbisdiphenylphosphinoxanthene; Xantphos
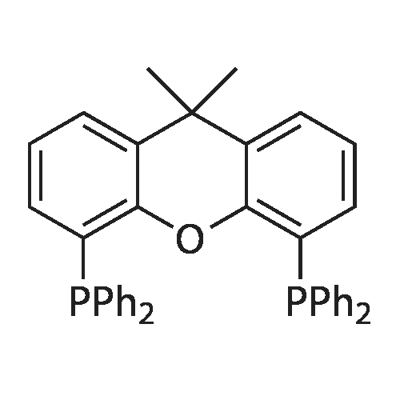

CAS No:161265-03-8
MF:C39H32OP2
Appearance:off-white or light-yellow crystalline powder
Specification:99.0%
Package:1/5/10/25kgs/drum
Supply Capacity:Tons
Synonyms:9,9-Dimethyl-4,5-bis(diphenylphosphino)xanthene; XANT PHOS;9,9-DIMETHYL-4,5-BIS(DIPHENYLPHOSPHINO)XANTHENE;(9,9-DIMETHYL-9H-XANTHENE-4,5-DIYL)BIS[DIPHENYL PHOSPHINE];4,5-BIS-DIPHENYLPHOSPHANYL-9,9-DIMETHYL-9H-XANTHENE;4,5-BIS(DIPHENYLPHOSPHINO)-9,9-DIMETHYLXANTHENE;Dimethylbisdiphenylphosphinoxanthene;9,9-Dimethyl-4,5-bis(diphenylphosphino)xanthene, XANTPHOS;4,5-BIS(DIPHENYLPHOSPHINO)-9,9-DIMETHYLX
4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene (Xantphos) is an organophosphine compound derived from the xanthene heterocycle. Recognized as a crucial bidentate ligand, its unique steric hindrance and electron-rich properties make it an essential reagent in organometallic chemistry for the efficient construction of complex chemical bonds, especially in challenging C-N and C-O coupling reactions.
Used as a ligand in cross-coupling reactions such as Buchwald, Suzuki, Negishi, Stille, and Heck.
Used in Buchwald–Hartwig aromatic amination; used as a ligand in Pd-catalyzed C-N cross-coupling reactions for synthesizing heterocycles (e.g., 3-bromothiophene with 2-aminopyridine).
Used in Ruthenium-catalyzed alkylation reactions of activated methylene compounds with alcohols; commonly used in the hydroformylation of olefins; complexes with transition metals are used as catalysts for carbonylation reactions.
Due to its anthracene skeleton and good electronic conductivity, it can be used as a component in conductive materials such as organic electronic devices and solar cells.
Organic reagent and pharmaceutical intermediate.